Reactive dyeing:
Reactive dyeing, a textile coloring process, actively interact with yarns and fabrics through a chemical reaction. Unlike traditional methods, where colors merely adhere to the surface, it creates brilliant and long-lasting colors. Reactive dyeing forms a permanent bond with the fabric, resulting in vibrant and enduring hues. This system incorporates transition molecules that facilitate a seamless connection between the dye and the fibers, ensuring not only a vibrant spectrum but also excellent colorfastness.

Necessity of reactive dyeing systems:
Till 1950, the dyes manufactured were not directly bonded to the fiber. Vat or Sulphur dyes required reducing and oxidizing process in addition. Direct dyes involved weak bonds only. Disperse dye, mainly for MMF, still by force swelling of fiber needed. Acid & Basic dyes introduced salt linkage & mordanting for cellulose. Azoic dye consists of multiple steps for color developing.
In 1955, Rattee and Stephen (ICI, UK), developed a dyeing system for cellulose with di-chlorotriazine group which ensured direct covalent bond.
Reactive dye included:
- Good wash & light fastness.
- Easy application.
- Cheap dye.
- High fixation & Band stability.
- Storage stability.
Reactive systems:
Four characteristic features in the reactive system.
- Chromophore grouping, contributing the color & much of the substantivity to cellulose.
- The reactive system, enabling the dye to react with the hydroxyl groups in cellulose.
- A bridging group, that links the reactive system to chromophore.
- One or more solubilizing groups, usually sulphonic or substituents attached to chromophore.
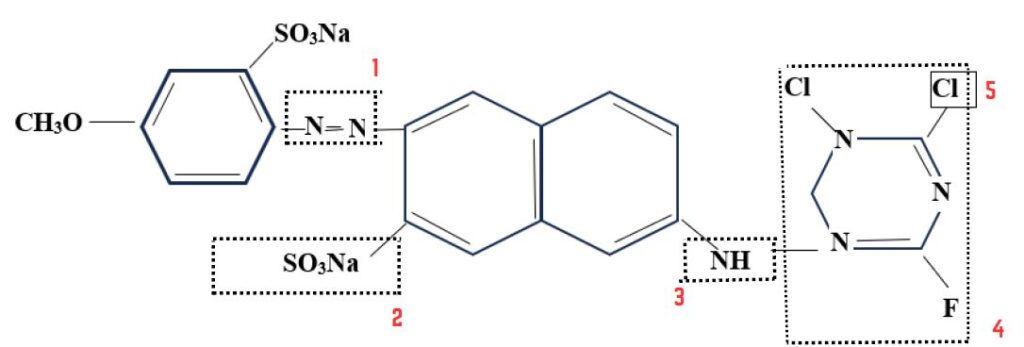
- Chromophore: N=N
- Reactive group:
- Leaving group: Cl, F etc.
- Solubilizing group: SO3Na
- Bridging group: -NH-
Reaction mechanism: Two types of reactions occur. They are-
- Nucleophilic substitution.
- Nucleophilic Addition.
- Nucleophilic substitution: Substitution of chlorine, fluorine, methyl sulphone or nicotinyl leaving group happens by an adjacent nitrogen atom in a heterocyclic ring.
- Nucleophilic Addition: Nucleophilic addition of cellulosate ion(cell-0–) and H+ ion to a C=C (carbon-carbon double bond) happens by an adjacent electron attracting sulphone group.
Reaction mechanism of Nucleophilic substitution:
Nitrogen containing heterocyclic rings bearing halogens substituents undergo nucleophilic substitution.
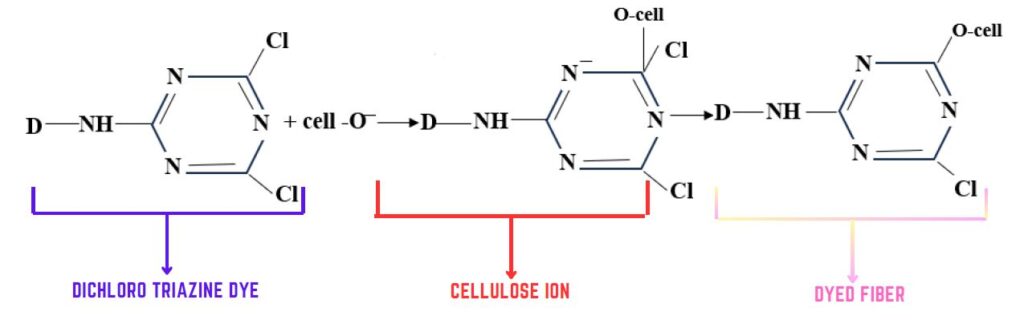
The hetero atoms in the aryl ring activate the system for nucleophilic attack because of their electronegativity.
- The attacking nucleophilic is cellulosate ion
- Thus fixation occurs.
Reaction mechanism of Nucleophilic addition:
Nucleophilic addition is applicable for VS(vinyl sulphone) compounds. Alkaline 1,2- elimination of Precursor (2-sulphato ethyl sulphone) is done to release the reactive vinyl sulphone system.
SO2-CH2-CH2O-SO3Na + NaOH→ -SO2-CH=CH2 + Na2SO4 + H2O
(sulphato ethyl sulphone) (Reactive vinyl sulphone)
During nucleophilic addition reaction, carbon-carbon double bond is polarized by the powerfully electron-attracting sulphone group. This polarization confers a positive character on the terminal carbon atom, favoring nucleophilic addition of cellulosate ion, leading to fixation.
Hydrolysis of reactive dye:
The fatal property of reactive dye is hydrolysis.
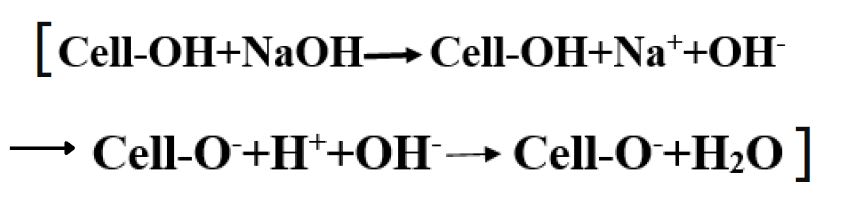
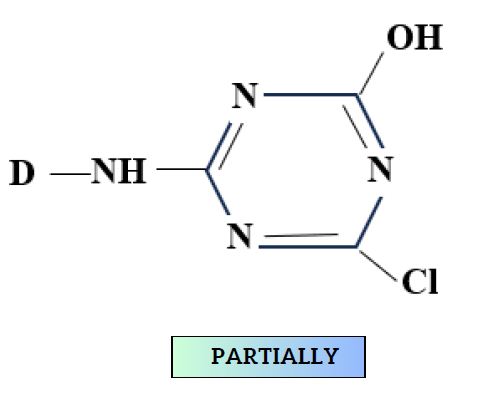
During Nucleophilic substitution, the nucleophile can be either cellulosate ion or hydroxide ion (from water).
During Nucleophilic addition,
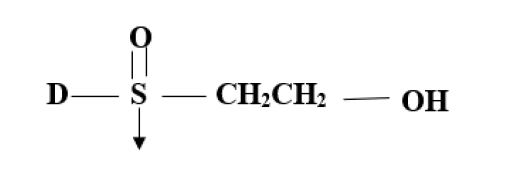
Classification of Reactive Dyes:
Reactive dyes can be classified according to different parameters. They are as follows:
Classification of Reactive Dye According to Reactive Group:
According to reactive group, Halogenated heteroycles & Activated Vinyl compounds Reactive dyes are available.
1. Halogenated heteroycles:
(a) Triazine group:
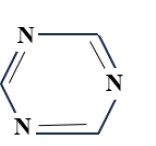
(B) Pyrimidine Group:
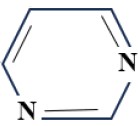
(C) Quinoxaline Group:
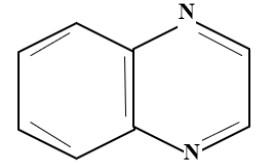
Levafix
2. Activated Vinyl compounds:
- Vinyl Sulphone (D-SO2-CH2-CH2-) Ex: Ramazol.
- Vinyl Sulphonamide (D-SO2-NH-CH2-CH2-) Ex. Levafix
- Vinyl acrylamide (D-NH CO-CH2-CH2-) Ex: Primazine
Classification of Reactive Dye According to reactivity:
- High reactivity : Ex: Procion M
- Moderate reactivity: Ex: Liva fix-E
- Low reactivity : Ex: Premazine
Classification of Reactive Dye According to Functional Group:
According to functional group, Monofunctional & Bifunctional Reactive dyes are available.
1. Monofunctional Reactive dye:
Contains only one possible reactive centre. Halogen substituent in the amino halo-triazine dyes or activated terminal carbon atom in the vinyl sulphone system. Examples are given below-
1. Di chlorotriazine dye:
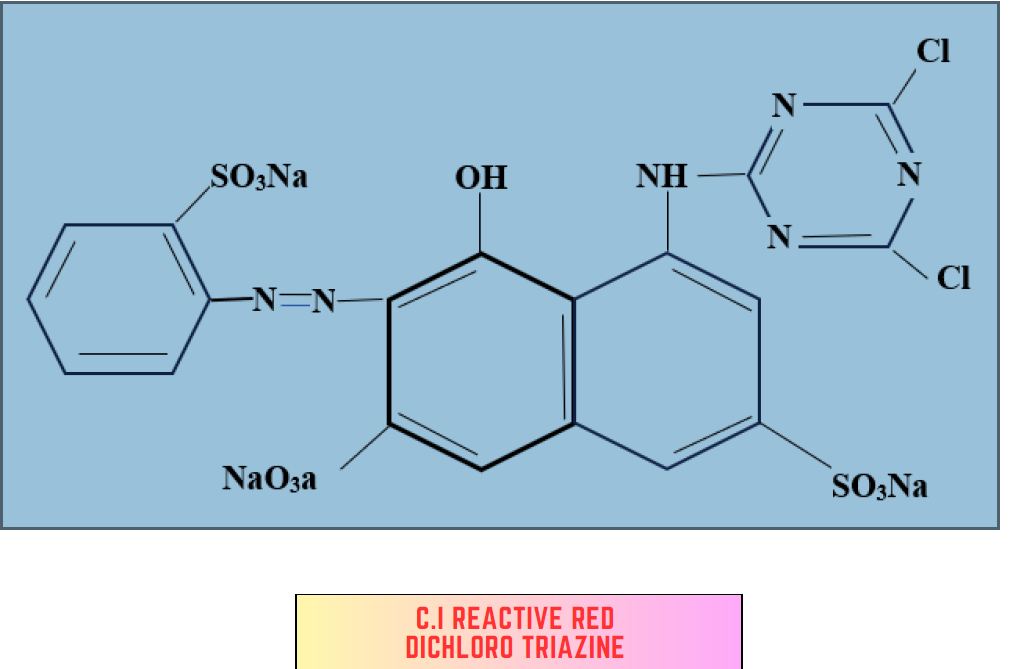
- Highly reactive
- Temp (30-40)°C
- Pad -batch dyeing
- Relatively small chromogens are preferred to ensure mobility.
- Suitable for bright dyeing.
- CI Reactive Red I
2. Amino-chloro-triazine dye:
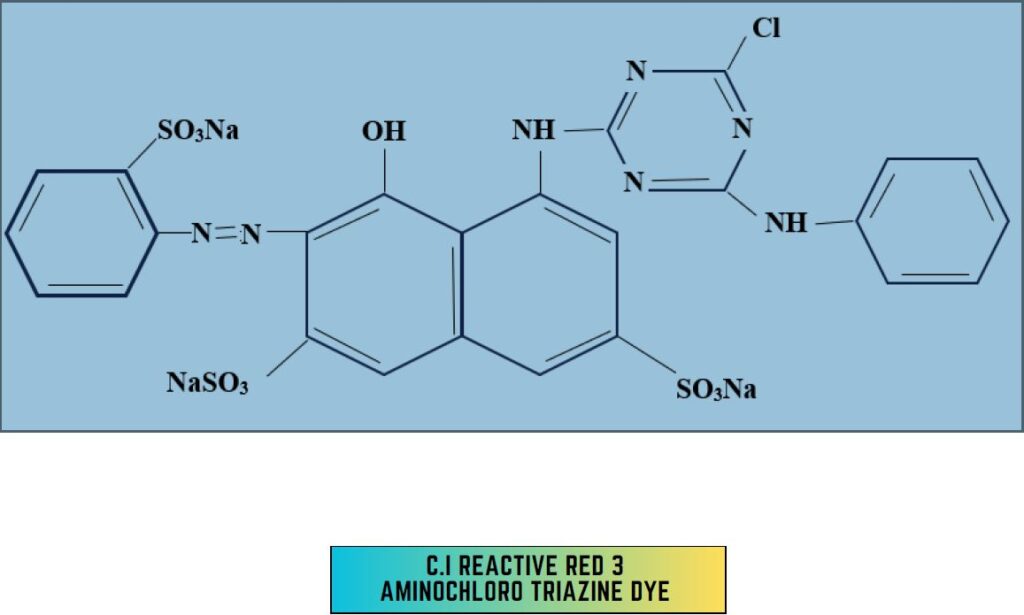
- 2-amino 4-chloro derivative
- Temp-80°C
- PH – 11
- CI Reactive Red 3
3. Amino-fluoro triazine dye:
- More reactive than Amino-chloro-triazine dye.
- Cibacron F (CGY)
4. Tri-chloro-pyrimidine dyes:

- Less activation of Chloro substituents.
- Temp – 100°C
- Stable bonding
5. Di-chloro-quinoxaline dye:
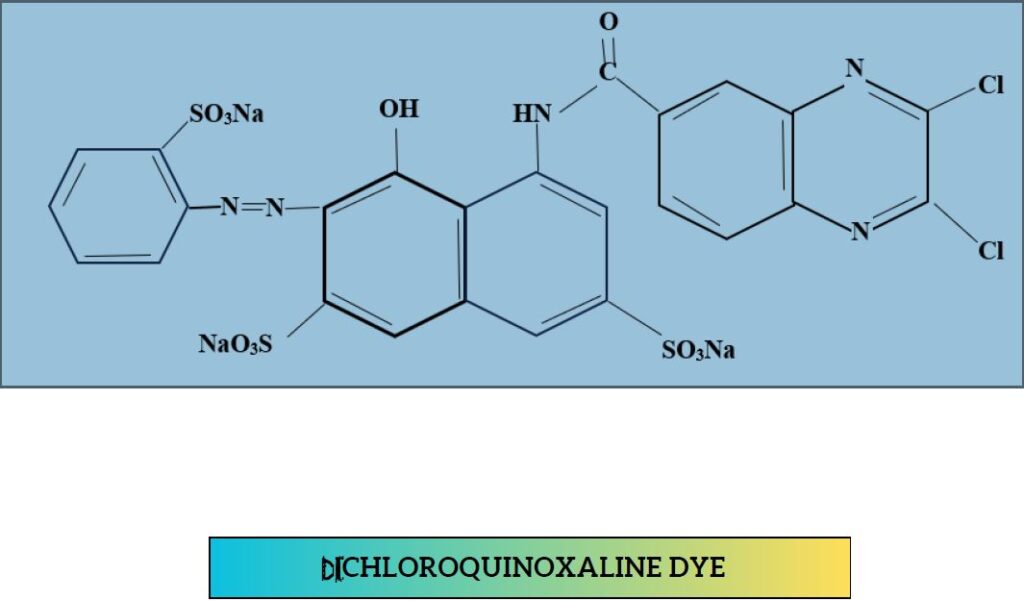
- Relatively higher than Di chloro-triazine & Di-fluoro-pyrimidine
- Temp-50°C
- Levafix E (BAY)
2. Bi-functional Reactive Dye:
From around 1970, concept on dyes of combined two reactive system developed. Some typical examples with criteria are given below-
1. Bis (Amino-chloro-triazine) dye:
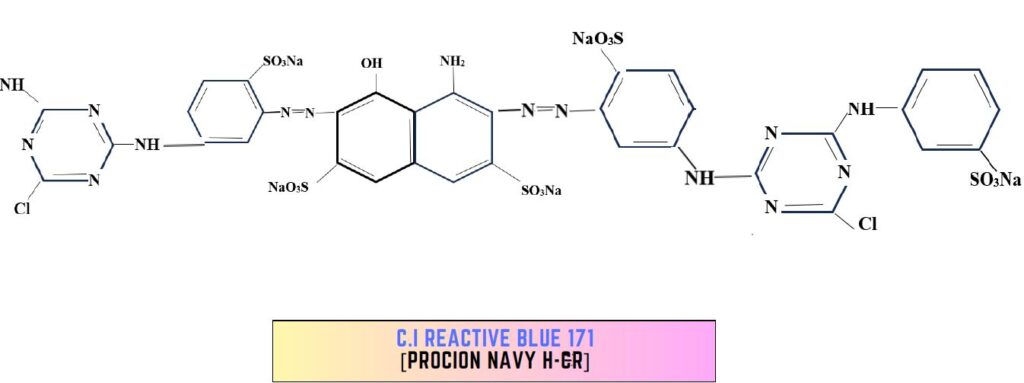
- Homo Bi-functional dye.
- High substantivity allows them to achieve excellent exhaustion at 80°C.
- Fixation 70-80%
- High temp application conditions ensure good good levelling.
- High fixation leads to:
- Better utilization
- Less hydrolyzation
- less discoloration of the element
- Rate of removal of unfixed dye is slow.
2. Bis (Amino-nicotino-triazine) dye:
- Homo Bi-functional dye.
- Amino-chloro-triazine + 3° amine
- Quaternary NH4 derivative.
- polarization of C-N increases.
- More reactive.
- PET/Cotton blend dying suitable, as >1000C dyeing recommended.
- N,N-dimethylhydrazine, pyridine.
- Kayacelon React Red CN-3B.
- Procion Red H-E3B
3. Aminochlorotriazine-Sulphatoethyl sulphone dye:
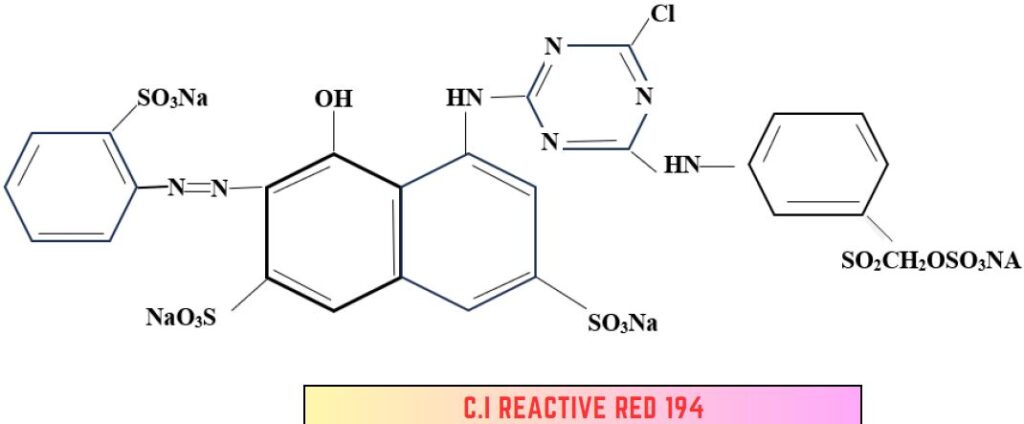
- Hetero Bi-functional dye.
- Di chlorotriazine dye + arylamine containing 2-sulphatoethyl sulphone → Bifunctional dye.
- Capable of reacting via mono-chloro-triazine moiety or a vinyl sulphone group.
- Sumifix Supra Brilliant Red 2BF (NSK).
- Vinyl Sulphone group ensures most of the fixation.
- Triazine bridging residue contributes higher substantivity.
- Temperature: 50-80°C
- Less affected by electrolyte and liquor Ratio.
- Low temperature suits VS (Vinyl Sulphone).
- High temperature suits CTR (Chlorotriazine).
Classification of Reactive Dye According to Control Parameter:
Group-1: Alkali controllable reactive dye:
- Temp of fixation: 40-60°C
- Low exhaustion before alkali add.
- Alkali addition influence high reactivity.
- Level dyeing to be taken care of by controlled dosage rate.
- Example: Di-chlorotriazine, Chloro-difluoro Pyrimidine, Di-chloro-quinoxaline, vinyl-sulphone etc.
Group-2: Salt controllable reactive dye:
- Temp of fixation: 80 – 100°C
- High exhaustion at neutral PH
- Level dyeing by adding salt. Portion wise or controlled rate of dosage.
- Low reactive.
- Example: Tri-chloro-pyrimidine, Amino-chlorotriazine etc.
Group-3: Temperature controllable reactive dye:
- Temperature greater than boil.
- Alkali not required.
- Self levelling.
- Time rise rate needs to control.
- Example: Kayacelon React (KYK) etc.
Factors governing reactive dye uptake:
- Linear density of fiber.
- Reactivity ratio.
- Substantivity ratio.
- Diffusion co-efficient of dye in the substate.
- Liquor ratio.
- Surface area of substrate available for absorption of dye.
Basie Principle of Reactive Dyeing with Steps:
- Dyeing commenced in neutral solution, often in presence of salt.
- Appropriate alkali added to increase PH, initiates dye-fiber reaction.
- -OH (hydroxyl groups) of cellulose are weakly acidic and absorption of hydroxide ion causes some dissociation, forming cellulosate ions, which react with dye by nucleophilic addition or substitution.
- Lower the reactivity, higher the temperature (hot brand) & higher the final PH
- Hydroxide ions also react with reactive group as like as cellulosate ion.
- This produces hydrolyzed dye.
- Although hydrolysis is slower, still affects fixation.
- Through washing requires for removal of unfixed and hydrolyzed dye.
- Rinsing (cold & warm), soaping, warm rinsing done.
Application options:
- Continuous – Pad-Thermofix.
- Semi continuous- Pad-Batch.
- Batch wise exhaustion – Winch, Jet, Package & Beam dyeing.
- Printing: Print-Thermofix.
Reference:
- Cellulosics Dyeing Edited By John Shore
- Basic Principles Of Textile Coloration – Arthur D. Broadbent
- Textile Preparation and Dyeing by A K Roy Choudhury
- Dyeing and Chemical Technology of Textile Fibers by E.R. Trotman




4 thoughts on “Reactive Dyeing in textile: mechanism, Classification and steps”
Sir
Very impressive article 👍
Appreciate your compliment Shihab.
This is a nice article sir💞
Thanks Abed for the compliment.