Disperse dyes:
Disperse dyes, which are organic coloring compounds that lack ionizing groups and possess low water solubility, are ideal for dyeing hydrophobic textile materials via colloid dispersion. This distinct class of dyestuff, suitable for synthetic fibers like polyesters, acetates, and polyamides, earns its name from its non-soluble, non-ionic nature with molecular dispersion. To enhance dispersal, additional chemicals are employed. The small size of the dye molecule is a defining characteristic of disperse dyes.
Properties of Disperse Dyes:
- Extremely low solubility in water.
- Used in finely dispersed state.
- Dye molecule held in fiber in molecularly dispersed state.
- Light fastness ranges from fair to good (rating 4-5).
- Sublimation power is notable, attributed to low molecular size, non-ionic nature, and the absence of sulphonated groups.
- Color fading occurs with heat application.
- Certain blue and violet disperse dyes with an anthraquinone dye structure fade in the presence of nitrous oxide.

Typical factors of disperse dyeing:
Factors influencing the dyeing performance of disperse dyes include:
- Raw material.
- Dye quality.
- Chemical class.
- Dispersing agent.
- Carrier.
- Temperature.
- pH.
- Time.
Dispersing agent:
Dispersing agents are crucial components effective under dyeing conditions and stable to challenges like hard water and high temperatures. They help maintain the dispersion of dye molecules in the bath. The dye is initially formed as relatively large particles, unsuitable for application on hydrophobic fibers. Utilizing these particles directly results in uneven and speckled dyeing, failing to achieve their full color value.
Functions of dispersing agents:
- Enabling the dye to be formed in powder form.
- Facilitating the reconversion of powder into a required dispersion when added to the dye bath.
- Maintaining fine dispersion throughout the dyeing process.
- Increasing the solubility of the disperse dye in water.
- Influencing the rate of dyeing.
- Assisting in particle size reduction of the dye.
Examples of dispersing agents:
- Soap powder
- Turkey red oil
- Alkyl sulphonates
- Alkyl acryl sulphonates
- Formaldehyde
- Lignin sulphonates
Typical Trade Names of dispersing agents:
| Manufacturer | Trade Name |
| Sandoz | Esalon |
| Hoechst | Hispogal |
| BASF | Setamol |
mechanism of disperse dyeing:
The mechanism involves the transfer of dye from a liquid solvent (water) to a solid organic solvent (fiber), particularly hydrophobic fibers like polyester. Disperse dyes are introduced to water with a surface-active agent, forming an aqueous dispersion. Their insolubility allows them to depart the dye liquor, exhibiting substantivity for organic fiber over inorganic dye liquor. The introduction of heat boosts the energy of dye molecules, increasing the dying of textile fibers.

The heating of the dye liquor induces fiber swelling, aiding the dye in penetrating the fiber polymer system. Subsequently, the dye molecules securely position themselves in the fiber’s amorphous regions through hydrogen bonds and Van Der Waals’ force. The dyeing of hydrophobic fibers, like polyester, unfolds in a sequential process through a three-stage mechanism:
1. Dispersion:
The initial stage involves the dispersion of dye into water, breaking up into molecules through dissolution. This process relies on the dyestuff’s disposability and solubility, further facilitated by dispersing agents and an elevation in temperature.
2. Adsorption:
After dispersing, the fiber surface actively adsorbs the dissolved dye. This adsorption process depends on the dye’s solubility both in the dye bath and within the fiber.
3. Diffusion:
The dye molecules actively diffuse from the fiber surface into the interior, determining the overall dyeing rate. Upon achieving equilibrium state, the following subsidiary equilibria emerge:
i) Dye dispersion in the dye bath – dye dissolved in the dye bath
ii) Dye dispersion in the dye bath – dye absorbed on the fiber surface
iii) Dye absorbed on the fiber surface – dye diffused in the fiber
iv) Dye diffused in the fiber – dye diffused from fiber to dye bath
Classification of Disperse Dye According to chemical constitution:
Azo dyes:
- Mono-Azo dyes:
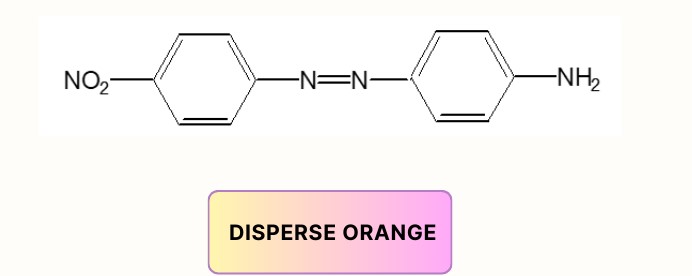
- Di-Azo dye:

Anthraquinone dyes:

Aminoketone dyes:

nitro dyes:

Application methods of disperse dye:
There are some typical application methods for disperse dyeing. These includes-
- Thermosol method (180° – 220°C)
- Carrier method (80° – 100°C)
- High temperature method (130° – 140°C)
1. Thermosol method:
Typical Recipe For Thermosol Method:
| Dye | x% OWF(On the Weight of Fabric) |
| Wetting agent | 1-2 gm/l |
| Acetic acid | 1-1.5 gm/l |
| Thickener | 20-40 gm/l |
| pH | 4.5-5 |
| Time | 2 hours |
Process sequence for Thermosol method:

mechanism of thermosol method:
In the thermosol process, the dye dissolves directly into the fiber using heat instead of a aqueous medium. The dye is deposited on the fiber surface, and exposure to dry heat at a temperature of approximately 220°C leads to the direct dissolution of the dye into the fiber. Complete penetration is achieved within 60 seconds.
Procedure Of thermosol method:
- Apply the dye solution onto the fabric through padding using the provided recipe.
- Dry the fabric at 100°C by means of hot flue or infrared(IR), adjusting based on the dryer used; excessive temperature may hinder solid shade formation.
- Fix the dyes at (180°-220°)C for 60-90 seconds, dependent on the fabric type, dye, and desired shade depth.
- Wash off unfixed dyes and chemicals with warm water.
- Perform soap washing or reduction clearing if necessary, as before.
- Conclude the process by washing the fabric and allowing it to dry.
Reduction Clearing:
A clearing process of polyester dyeing employing heavy reduction (NaOH+Na2S2O4) actively washes away non-penetrated, adsorbed dye.
Typical Recipe For Reduction Clearing:
| Sodium Hydrosulphite or dithionite (Na2S2O4) | 2 gm/l |
| Caustic Soda (NaOH) | 1-2 gm/l |
| Detergent | 1 gm/l |
| M:L | 1:30 or 1:50 |
| pH | 4.5-5 |
| Time | 30 minute |

Typical Recipe For Soaping:
| Soap | 1 gm/l |
| M:L | 1:30 or 1:50 |
| Temp | 60°C |
| Time | 30 minute |
Pros and Cons Of Thermosol method:
Pros:
- Short dyeing time.
- Elimination of the need for a carrier.
- Absence of additional issues related to carrier removal using alkali.
- Attainment of very bright shades.
- Excellent dye utilization (75-90%).
- Non-toxic.
Cons:
- Necessity for specialized machinery.
- Risk of strength loss with prolonged treatment.
- Potential change in shade due to sublimation at high temperatures.
- Increased cost due to the special arrangements required for this process.
2. Carrier Method:
Carrier:
Carriers are dyeing assistants which alter the dispersing properties of the dyes and physical characteristics of the fibre so that more of the dye can be transferred from the dye bath to the fibre than in the absence of these assistants. It is a are kind of organic compound that acts as a substantive swelling agents. In case of hydrophobic fiber such as polyester fiber carrier is added to dye bath or print paste for increase of dye take up.
Selection of Carrier:
Considerations for carrier selection include the following factors-
- Market availability.
- Non-toxicity.
- High efficiency.
- Cost-effectiveness.
- Minimal impact on light fastness.
- No fiber degradation or discoloration.
- Stability under dyeing conditions.
- Compatibility with dyestuffs.
- Easy dispersion in the dye bath.
- Straight forward removal after dyeing.
mechanisms of Carrier:
- Inducing fiber swelling and relaxation.
- Formation of a dye film on the fiber surface.
- Transporting dye to the fiber through dye-carrier association in the bath.
- Increasing dye solubility.
- Enhancing the diffusion rate of products with hydrophilic groups in polyester fibers.
- Augmenting fiber swelling.
- Improving dye uptake through covalent bonding with fiber liquid.
- Potentially acting as molecular lubricants.
- Penetrating the fiber polymer chain, reducing inter-chain attraction, and facilitating dye molecule entry into the polymer structure.
Typical Recipe For Carrier Method:
| Dye | x% OWF(On the Weight of Fabric) |
| Carrier | 1-4% OWF(On the Weight of Fabric) |
| Wetting agent | 1-2 gm/l |
| Dispersing agent | 0.5-1% |
| Acetic acid | 1-2 gm/l |
| M:L | 1:10 |
| pH | 4.5-5.5 |
| Temperature | 100°C |
| Time | 1 hour |
Procedure Of Carrier method:
Dyeing Curve For Carrier Method:

- Prepare the dye sol with cold water (1:10) and let it stand for 15 minutes.
- Set the dye bath at 60°C and sequentially mix carrier, dispersing agent, and salts.
- Add the material and maintain it for 15 minutes without increasing the temperature.
- Introduce the dye sol, and regulate the pH with CH3COOH.
- Increase the temperature to 100°C, then proceed with dyeing 1 hour.
- Reduce the temperature to 70°C, followed by rinsing and potential reduction clearing.
Reduction Clearing:
A clearing process of polyester dyeing employing heavy reduction (NaOH+Na2S2O4) actively washes away non-penetrated, adsorbed dye. Reduction clearing is specifically applied for medium and deep shades to enhance wash fastness.
Typical Recipe For Reduction Clearing:
| Sodium Hydrosulphite or dithionite (Na2S2O4) | 2 gm/l |
| Caustic Soda (NaOH) | 1-2 gm/l |
| Detergent | 1 gm/l |
| Temperature | 70-80° C |
| Time | 30 minute |
Pros and Cons Of Carrier method:
Pros:
- Enables dyeing in simple equipment under atmospheric pressure at temperatures up to 100°C for polyester fiber.
- Provides moderate-level dyeing in polyester dyeing.
- Shortened dyeing cycles due to accelerated dyeing.
- Enhanced fastness properties resulting from increased penetration into the fiber.
- Accelerates the rate of dyeing.
- Certain carriers reduce wool staining when dyeing polyester/wool blends.
- Improves leveling.
Cons:
- Higher cost.
- Reduced light fastness.
- Toxicity associated with some carriers.
- Some carriers may cause spotting issues.
- The removal of the carrier using alkali adds to the overall cost.
- Odor and air pollution issues.
3. High temperature Method (HTM):
Typical Recipe For High temperature Method:
| Dye | x% OWF(On the Weight of Fabric) |
| Salt | 0.5-1% |
| Dispersing agent | 0.5-1% |
| Acetic acid | 1 gm/l |
| M:L | 1:8 |
| pH | 4.5-5.5 |
| Temperature | 130°C |
| Time | 1 hour |
Procedure Of High temperature method:
Dyeing Curve For HT Method:

- Prepare dye solution by adding cold water (1:8) and let it sit for 15 mins.
- Set dye bath at 60°C, adding dispersing agent and salt.
- Treat the material for 15 minutes without raising the temperature.
- Add the dye solution and control the pH with CH3COOH.
- Raise the dye bath temperature to 130°C within 30 minutes.
- Continue dyeing at 130°C for 1 hr.
- Cool the dye bath as quickly as possible.
- Allow the fabric to undergo hot rinsing.
- Perform reduction clearing if required, as before.
- Rinse the fabric again and then dry.
Reduction Clearing:
A clearing process of polyester dyeing employing heavy reduction (NaOH+Na2S2O4) actively washes away non-penetrated, adsorbed dye. Reduction clearing is specifically applied for medium and deep shades to enhance wash fastness.
Typical Recipe For Reduction Clearing:
| Sodium Hydrosulphite or dithionite (Na2S2O4) | 2 gm/l |
| Caustic Soda (NaOH) | 1-2 gm/l |
| Detergent | 1 gm/l |
| Temperature | 70-80° C |
| Time | 30 minute |
Pros and Cons Of High temperature method:
Pros:
- Most common method.
- Shorter dyeing times.
- No need for a carrier.
- Dye transfer from liquid.
- Up to 98% dye fixation.
- Minimal dye loss.
- Solvent (water) to solid organic solvent (fiber).
- Typically higher light fastness and wet fastness.
- Improved exhaustion and deeper dyeing achievable.
- Faster diffusion of the dye in the fiber at elevated temperatures.
Cons:
- Higher temperature requirements.
Heat setting:
The process, which induces dimensional stability to fiber, yarns, fabrics or garments with successive heating and cooling in dry and moist conditions is called heat setting. Heat setting is an important sequence in manufacturing process of synthetic fiber. To know more about manufacturing process of synthetic fiber, you can go through the article entitled “Synthetic Fibers: Manufacturing Process And Classification”.
The different levels of heat setting may be defined as:
- Temporary set.
- Semi-permanent set.
- Permanent set.
Importance of Heat setting procedures for polyester:
Heat setting procedures for polyester fabric are crucial for various reasons:
- Modifying crystalline structure.
- Improving dimensional stability.
- Increasing safe ironing temperature.
- Avoiding shade variation.
- Resisting wet creasing during washing.
- Affecting water absorbency.
Reference:
- Basic Principles Of Textile Coloration – Arthur D. Broadbent
- Dyeing and Chemical Technology of Textile Fibers by E.R. Trotman
- Chemistry of dyes and principles of dyeing by Shenai V.A.
















9 thoughts on “Disperse dyes: Properties, Mechanisms, and Application Methods in Textile Industry”
great 🥰🥰🥰
Thanks for the compliment dear Mahbub.
Important topics
Thanks Hasnain.
Wow that’s good article 👏
great article
Thanks for support.
Great work sir🥰🥰
Thanks Alif